💬 Research Question of the Month
In 2024 we plan to invite more scientists and clinicians from outside the 8p research network to present at our monthly research roundtable. What topics would you like to see covered? Do you know someone we should invite? Let us know in the comments!
🔬Research Highlight
As 2023 wrapped up, so did the first Project 8p-Sponsored Research Project. The project, which was led by Dr. Nicoleta Moisoi at De Montfort University, and carried out by post-doctoral researcher Dr. Marta Domínguez-Prieto, began in July 2022, when we sent 4 fibroblast cell lines from 8p heroes across the pond to their lab in the UK.
Mitochondrial dysfunction is a hallmark of neurodegenerative disease and we sought to understand if this is also the case in Chromosome 8p disorders. Fibroblasts are a good model system for studying cellular physiology because metabolic changes observed in neurons are often preserved in fibroblasts, yet they are easier and more cost-effective to work with than neurons (Olesen et al. 2022, Cordone et al. 2020). The first phase of the project focused on phenotyping mitochondrial function and cellular features of the fibroblasts. The experiments showed that 8p cells exhibit differences in mitochondrial resting membrane potential, lysosomal staining, staining of the plasma membrane and ER membrane, and cellular senescence. You can read more about those results on the Chromo 8p Substack. Further work will be necessary to understand how these changes may contribute to disease pathology, but in the meantime, we can take advantage of these differences to screen for drug candidates capable of correcting the morphological phenotypes of 8p cells such that they look more like the control cells.
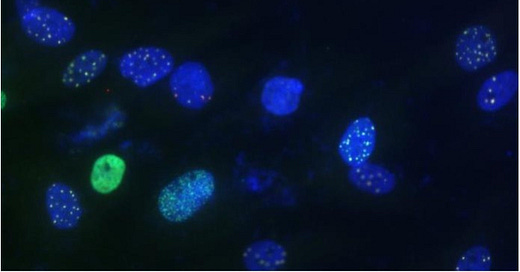
The second phase of the project, which was completed in December, aimed to assess the DNA damage and repair response in 8p fibroblasts, as accumulation of DNA damage is another common feature of neurodegeneration. To induce DNA damage, cells were treated with Bleomycin. They were then stained for the DNA double-strand break repair proteins H2AX and 53BP1 to assess their response. The image above shows the stained cells after treatment with 40 μg/mL of Bleomycin (Hoechst, blue, nuclear stain; γH2AX, red; 53bp1, green).

In glucose media, two of the 8p lines accumulated fewer marker proteins (8p 1 and 8p 2), while the two accumulated similar levels as the controls (8p 3 and 8p 4). In galactose media, which enhances metabolism by mitochondrial respiration, all of the 8p lines showed a reduced response to Bleomycin treatment. The reduced accumulation of DNA damage/repair markers could mean that the cells are resistant to DNA damage, or that they are impaired in targeting the repair proteins to sites of DNA damage. These results are consistent with our earlier results (i.e., increased autophagy, lower mitochondrial membrane potential, enhanced senescence), which suggest that cellular stress is driving 8p cells into a state of senescence. Could these changes also happen in brain cells? If so, drugs that are able to alleviate the stress of aneuploidy in skin cells might also promote healthy neuronal development and function.
📄 Recent Articles and Publications
Let us know if you’ve read or published a paper or article recently that you think the 8p community should know about!
Chromosome 8p engineering reveals increased metastatic potential targetable by patient-specific synthetic lethality in liver cancer. Huth, T. et al. Sci. Adv. Dec 2023. https://www.science.org/doi/10.1126/sciadv.adh1442
Deletions on chr8p are common in many cancers and correspond with poor prognosis. To study the effects of chr8p loss of heterozygosity on gene expression and metastatic potential, the authors used CRISPR-Cas9 to generate liver cancer cell lines with heterozygous deletion of a 33 Mbp region of chr8p (chr8pLOH). Furthermore, they performed a synthetic lethal CRISPR screen of the chr8pLOH lines, which identified NUDT17 (Chr 1) as a synthetic lethal paralog of the 8p gene NUDT18. The authors propose that these two proteins function redundantly to clear 8oxoG and show that loss of NUDT18 sensitizes cells to loss of NUDT17. As wild-type cells are minimally affected by loss of NUDT17, the gene could be used as a therapeutic target to specifically inhibit the growth of cancer cells harboring chr8p deletions.
Interestingly, NUDT18 is within the region that is often duplicated in Chr 8p disorders, suggesting that such cells may actually have an enhanced ability to respond to oxidative stress.
Constructing human neural circuits in living systems by transplantation. Pașca, SP. Cell. Jan 2024. https://www.sciencedirect.com/science/article/pii/S0092867423013405?dgcid=author
Human stem cell-derived cells transplanted into rodent brains allow the study of complex neural circuits not formed in in vitro models. This review describes the current approaches, applications, and limitations of such xenotransplantation techniques.
Building synthetic chromosomes from natural DNA. Coradini, ALV et al. Nat. Commun. Dec 2023. https://www.nature.com/articles/s41467-023-44112-2
This article describes a new method for constructing synthetic chromosomes from natural DNA components, as opposed to traditional de novo assembly methods, which involve the synthesis of many DNA fragments. The method, named CReATiNG (Cloning, Reprogramming, and Assembling Tiled Natural Genomic DNA), involves cloning fragments of chromosomes and assembling them in yeast through homologous recombination. CReATiNG can be used to study the effects of genome structure and copy number by synthesizing chromosomes with inversions, duplications, and deletions. The method is cheaper and faster than existing methods, and the synthetic chromosomes generated can replace the native chromosomes in cells. Further development is likely to extend this method to mammalian DNA.
💜 Family Corner
Participation Checks Available!
Unsure of your status in the My Hero Initiative?
Email kaiti@project8p.org and add your piece to the puzzle today!
📆 Upcoming Events
Research Roundtable with the Multidisciplinary Neurogenetics Clinic, Children’s Hospital Colorado. January 22, 2024 1:30 pm EST on Zoom. Email whitney@perlara.com to attend.